|
The research described in this newsletter is supported as part of the
Biological Electron Transfer and Catalysis, an Energy Frontier Research Center funded by the
U.S. Department of Energy, Office of Science.
|
BETCy breakthrough featured in Nature Chemical Biology
Paper describes flavin-based electron bifurcation in Nfn
Flavin-based electron bifurcation has recently gained acceptance as a fundamental mechanism of biological energy conservation. By leveraging a multitude of biophysical techniques across five of BETCy's labs, the energy landscape by which bifurcation occurs in the transhydrogenase enzyme, Nfn, was elucidated.
An extremely short-lived flavin intermediate (10 picoseconds) was observed for the first time and was key to unraveling the mechanism of bifurcation at unique flavin sites. This short lifetime is a requirement that ensures electrons are transferred to the endergonic branch within Nfn so that the high-energy chemical reaction occurs. The integrated nature of BETCy helped drive these technical advances, highlighting the more than one volt of electrochemical potential that Nfn generates during the catalytic mechanism. This unprecedented range of thermodynamic driving force accounts for the unique chemical reactions that are catalyzed by bifurcating enzymes.
With this fundamental knowledge, BETCy is advancing our understanding of energy transfer and catalysis, supporting the realization of biologically inspired complexity and functionality with earth-abundant materials to transform energy conversion, transmission and storage technologies through a detailed understanding of energy conservation mechanisms.
|
 |

|
BETCy Scientists Describe Electron Transfer Pathway in Cpl Hydrogenase
|
BETCy scientists from
NREL, MSU, ASU
, and
UGA
published a new paper focused on spectroscopic characterization of CpI, a model [FeFe]-hydrogenase that functions physiologically to produce hydrogen. The article, "The reduction potentials of [FeFe]-hydrogenase accessory iron-sulfur clusters provide insights into the energetics of proton reduction catalysis," appeared in the June 21, 2017 issue of the
Journal of the American Chemical Society
.
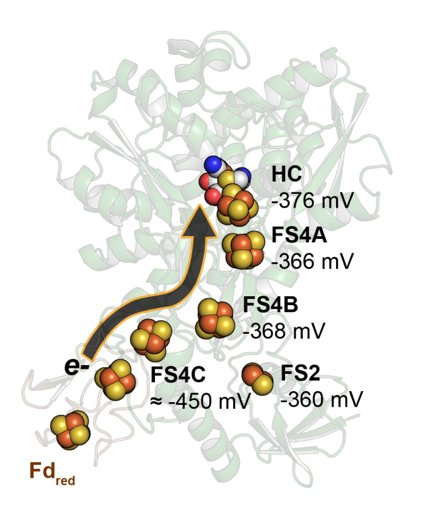
CpI houses a branched electron transfer pathway comprised of an array of FeS clusters, which deliver electrons from the physiological electron donor, ferredoxin, to the active site. The lead author of the study, Jacob Artz in the Peters Research Group said, "We used electron paramagnetic spectroscopy to identify the properties of each of the FeS clusters, and this enabled us to determine how they influence catalytic turnover of CpI."
EPR is sensitive to unpaired electrons, such as those in the FeS clusters in CpI.
Artz further explained that the EPR spectra were highly complex, with many overlapping signals that had to be deconvoluted through the use of a truncated CpI protein variant and a series of simulation steps. Using this methodology, the team was able to measure the individual reduction potentials in the array of FeS clusters in the CpI enzyme.
This analysis allowed for the development of a model that shows the exquisite tuning of these clusters, where the FeS cluster furthest from the active site has a very negative reduction potential (~ - 450 mV vs SHE), allowing the electron to travel through the pathway to the active site with no energetic barriers. The branch point of the pathway serves to influence the nearby FeS cluster. These insights serve to broadly inform how catalysis and electron transfer processes may occur in enzymes with complex electron transfer pathways.
This publication represents a major effort by the BETCy team, sourcing contributions from four different labs. Many of the experiments required key personnel to meet in-person at the NREL Spin Resonance Center in order to meld their collective talents and expertise into scientific results. The team is thrilled about the capabilities developed for this project, which will translate into the ability to understand even more complex systems within BETCy, and will pave the way for studying key mechanistic features of bifurcating hydrogenases.
|
 BETCy Promotes Synergy Between Laboratories with its ETC Innovation Award
The BETCy EFRC uses an internal travel award program as one mechanism to promote the development of innovative experimental research while
taking advantage of the unique technical capabilities throughout the center's
research laboratories.
The program, termed the BETCy ETC Innovation Award, for Exploiting Technical Capabilities, solicits proposals from graduate students and other key personnel within the center with the goal to encourage high-risk/high-reward research that exploits the diverse areas of expertise found within the 12 research groups of BETCy. Winning applicants travel to other BETCy laboratories for hands-on learning of new techniques under the mentorship of another BETCy PI. The program helps to synergize interactions between BETCy laboratories in the pursuit of novel avenues of investigation.
 Natasha Pence, a graduate student in the Peters laboratory, was an ETC Innovation awardee and travelled to Tempe, Arizona to work on developing a novel technique to measure binding affinity using protein film electrochemistry (PFE) with Prof. Anne Jones at Arizona State University. Natasha Pence, a graduate student in the Peters laboratory, was an ETC Innovation awardee and travelled to Tempe, Arizona to work on developing a novel technique to measure binding affinity using protein film electrochemistry (PFE) with Prof. Anne Jones at Arizona State University.
Ms. Pence explains the rationale for her winning ETC proposal: "The objective of my project was to use PFE to measure the binding affinity for the nitrogenase Fe protein in its different nucleotide-bound states (MgATP- or MgADP-bound) and its physiological reductant flavodoxin." Her project is part of BETCy's effort to understand how nitrogenase utilizes nucleotide driven electron transfer to combine chemical bond energy with electrochemical potential to drive difficult chemical bond forming reactions. Detailed knowledge of the interactions between flavodoxin and the different nucleotide-bound states of the Fe protein is important for a fundamental understanding of the Fe protein cycle.
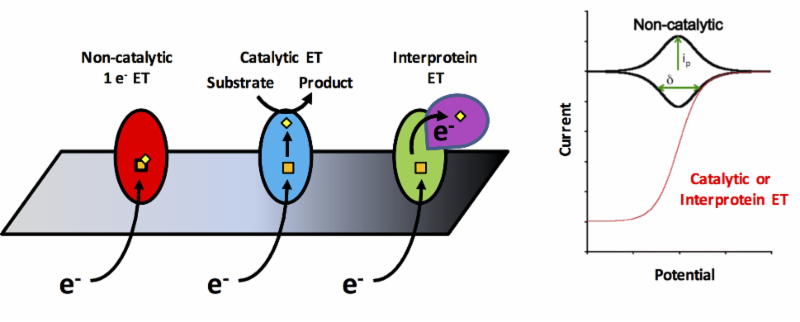 PFE involves adsorbing a redox active enzyme onto an electrode and monitoring direct electron transfer using electrochemical methods. An advantage over traditional solution methods is that the operating potential can be continuously swept to provide the driving force to initiate catalysis. Furthermore, changes in the catalytic turnover directly relates to change in electrical current, so activity can be measured over time. PFE involves adsorbing a redox active enzyme onto an electrode and monitoring direct electron transfer using electrochemical methods. An advantage over traditional solution methods is that the operating potential can be continuously swept to provide the driving force to initiate catalysis. Furthermore, changes in the catalytic turnover directly relates to change in electrical current, so activity can be measured over time.
While in Tempe, Ms. Pence successfully adsorbed flavodoxin onto an electrode using both covalent and non-covalent methods. Her preliminary data were promising, as reduction was observed when the Fe protein was titrated in the working solution. She says, "Having just started my graduate studies as well as being new to the BETCy team, I appreciated the opportunity to learn a new technique as well as meet and work with my fellow BETCy collaborators that are outside of my direct thrust. The ETC award provided a unique and valuable experience that is only possible with being a member of a multi-lab research consortium like BETCy."
|

 BETCy Scientists Participate in EFRC Early Career Network BETCy Scientists Participate in EFRC Early Career Network
The EFRC Early Career Network (ECN) is a platform established by the DOE to foster networking among early career scientists in the DOE through a series of virtual and face-to-face meetings. Each year the DOE asks the EFRC directors to nominate early career scientists from their center to serve as representatives on the ECN. Several individuals from BETCy research groups have participated.
 |
|
 |
Cara Lubner |
The first BETCy representative on the ECN was
Cara Lubner, now a staff scientist at NREL in BETCy's King Research Group. In describing her experience on the ECN, Dr. Lubner says, "I found it interesting to hear about the perspectives and challenges of other early career scientists. It was also quite refreshing to interact with scientists at other EFRCs and learn about how other Centers are structured and organized. Often times at conferences we focus on the science being performed, but the ECN gave us an opportunity to compare notes on the day-to-day operations of an EFRC. I was surprised to hear how diverse each person's experience was, particularly pertaining to the level of involvement within their EFRC group. The ECN experience gave me a greater appreciation for the opportunities that BETCy has given us as early career scientists, as well as a broader perspective by which to engage with my fellow colleagues."
 |
|
|
|
Rhesa Ledbetter
|
Rhesa Ledbetter, a graduate student in BETCy's Seefeldt Research Group, was a representative on the ECN during the 2016-2017 term. She says, "The ECN not only helps foster relationships with other early career scientists, but also offers opportunities for professional development in the form of workshops focused on topics such as grant writing and presentation skills." The workshops often have a panel of experts, each who share their knowledge and experiences on a given topic. "Learning from others is invaluable--the variety of perspectives is inspiration that I will use as I move forward in my career."
 |
|
 |
Jacob Artz
|
Currently, BETCy's representative on the ECN is Jacob Artz, who is a postdoctoral fellow in the Peters Research Group. Artz has found his ECN tenure rewarding. "It's been exciting to be introduced to the other representatives and their centers. The EFRCs tackle some very difficult science, and it's exciting to participate in that process." He looks forward to attending this year's DOE meeting in Washington, DC, which will feature the representatives from across the EFRCs, as well as the Energy Innovation Hubs and Computational Material Science Centers. "Being the ECN representative for BETCy has allowed me to gain a valuable perspective on the breadth of chemistry being studied, and I look forward to future collaborative opportunities with the other young professionals."
At the upcoming meeting in Washington DC, the Early Career Network has made plans for several events, including a tour of the Air and Space Museum, panel discussions for Career Paths for Young Professionals, Diversity in Energy Science and Future Energy Needs, and attending a baseball game at Nationals Park.
|
 New BETCy Technical Capability for Probing Biomolecular Interactions
New BETCy Technical Capability for Probing Biomolecular Interactions
|
|
|
Figure adapted from Jerabek-Willemsen, M. et al. (2014).
|
The Peters Research Group recently acquired a new instrument that will enable BETCy scientists to probe biomolecular interactions in new ways. The Monolith NT.115 instrument uses microscale thermophoresis (MST) to quantify interactions between biomolecules.
MST measures molecular motion along a thermal temperature gradient to detect changes in their hydration shell, charge, and size. The instrument uses an infrared laser to induce a temperature gradient in the sample, and the molecular motion is quantified optically through covalently attached dyes or fluorescent fusion proteins. The MST methodology is scalable and can detect interactions from ions and small molecules to large multi-protein complexes. The Monolith NT.115 is unique in that it combines the sensitivity of MST with the precision of fluorescence detection to measure interactions. The broad application range for this method can provide information on multifaceted processes such as complex formation, assembly order, and cooperativity.
For BETCy, quantifying protein-protein interactions for oxygen sensitive enzymes such as nitrogenase and hydrogenase has been a key technical challenge. Typically, this requires that the instrument be placed inside an anaerobic chamber to protect the FeS clusters within these enzymes from oxidative damage. Using the new MST instrument greatly simplifies analysis of oxygen sensitive samples because the samples can be sealed in capillaries inside an anaerobic chamber and then removed from the chamber and analyzed on the benchtop. This saves valuable space in the anaerobic chamber and enables investigation of interactions for both oxygen sensitive and aerobic systems, thereby permitting greater experimental flexibility.
Initially, the Peters Group plans to use the MST instrument to probe protein-protein interactions between flavodoxin and the nitrogenase Fe protein. Using the oxygen sensitive nitrogenase system as a platform to test-drive the MST technology will enable the Peters group to incorporate the binding affinities from the MST experiments with biochemical data from other BETCy research groups to provide insight into the order between nucleotide exchange and re-reduction of the Fe protein, the final steps of the nitrogenase Fe Protein cycle.
Peters says, "In the past, it has been difficult obtaining binding affinity data for nitrogenase and other oxygen sensitive enzymes, but hopefully with this new MST technical capability, these types of technically demanding experiments will become easier."
|
 |
|
|
|