DART Technologies at the SOFT/TIAFT in Boca Raton
|
|
 |
We invite you to visit our booth to discuss our
PARALLEL SPME technology and meet up with our new VP of Sales Darlene Murphy. In our booth we present the DART/QDa mass detector and PIMISA, our semi-automated software for rapid determination of the presence of drugs of abuse. We can also show you our
SPE-it kit which enables simultaneous solid phase extraction of up to 96 samples in under an hour. Ask us about joining our
SPME-TM validation program for rapid drug screening of urine. This exciting new technology is described in detail in the feature article from WATERLOO University cited below.
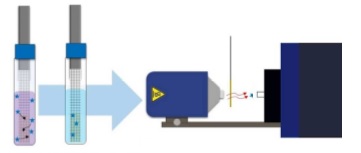
Rapid Extraction by using SPME-TM Coupled to DART-MS analysis
Imagine screening a hundred samples an hour!
Check out this year's posters and talks by DART users at SOFT below! Download the Scientific Sessions program schedule and list of abstracts here for more info.
Tuesday 12 PM Session A: Emily Barrey, Millipore Sigma
Using Direct MS and Microextraction for Drug Screening from Biological Matrices
Tuesday 4 PM Session 4B: Haley Mulder, Virginia Commonwealth University
Dripping Meets Dabbing: Cases Using the Rebuildable Dripper Atomizer to Vape Non-Traditional E-liquids and Drugs-Other-Than-Nicotine (DOTNs)
Wednesday
12 PM Session A: Masaru Kondo, Bureau of Health & Welfare (Japan)
Rapid Detection of Illegal Drugs in Urine by MEPSTM-DART-TOF-MS Analysis
Thursday
12 PM Session A: Julia Grzymkowski, Virginia Commonwealth University
Identification of "Kratom" (Mitragyna speciosa) Alkaloids in Commercially Available Products
Brittany Laramee
Product Specialist
|
|
|
|
On-site analysis of complex matrices by SPME-transmission mode coupled to a portable mass spectrometer via DART
Gómez-Ríos, Vasiljevic, Gionfriddo , Yu, Pawliszyn
Department of Chemistry, University of Waterloo, Waterloo, Ontario, Canada
The on-site screening for target analytes in complex matrices, not only requires reliable and portable analytical instrumentation, but also solvent-free and easy-to-use sampling/sample preparation approaches that allow analytes of interest to be isolated from such matrices. In this study, we evaluated transmission mode SPME coupled to a compact single quadrupole MS system, via DART, as a tool for the rapid screening of target analytes. Limits of quantitation (LOQ) ≤ 50 ng/mL were attained for the analytes with total analysis times under 2 min per sample. We demonstrated the rapid identification of milk samples from assorted animal and vegetal sources.
|
Species Identification of Necrophagous Fly Eggs Revealed by Direct Analysis in Real Time Mass Spectrometry
Justine E Giffen, Jennifer Y. Rosati, Cameron M Longo, and Rabi Ann Musah
Department of Chemistry, University at Albany, New York, USA
& John Jay College of Criminal Justice, New York, USA
Colonization of decomposing remains by necrophagous insects is of
forensic importance because the progression through stages of insect development can
be correlated to time of death.
We report here that the species identity of insect eggs can be determined from their chemical fingerprint signatures acquired by direct analysis in real-time high-resolution mass spectrometry (DART-HRMS). Freshly laid eggs were collected and distinguished from multiple necrophagous fly species. Multivariate statistical analysis of their observed DART-HRMS spectra revealed intraspecies similarities and interspecies differences in amino acid profiles that were the basis of species differentiation. The rapidity of the method makes feasible the generation of a fly egg chemical profile database against which the DART-HRMS spectra of unknown eggs can be screened in order to rapidly assess species identity.
|
Comparison of Ambient and Atmospheric Pressure Ion Sources for Cystic Fibrosis Exhaled Breath Condensate Ion Mobility-Mass Spectrometry Metabolomics
Zang, Pérez, Jones, Monge, McCarty, Stecenko, Fernández
Georgia Institute of Technology, Georgia, USA
Cystic Fibrosis breath metabolomics investigations via exhaled breath condensate (EBC) analyses could expose metabolic alterations associated with CF pathology and help assess the effectiveness of CF therapies. Transmission-mode DART-TWIMS-TOF MS was tested as a high-throughput alternative to electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI). In a comparison where EBC was collected from a control, ESI detected the most metabolites, APCI less, and TM-DART the least. TM-DART-TWIMS-TOF MS was used to profile metabolites in EBC samples finding that a panel of three discriminant EBC metabolites differentiated classes with excellent cross-validated accuracy.
|
 |
|
Visit
our partners at Cayman Chemical at their
Symposium on the opioid epidemic and join the conversation about the challenges faced by the first responders, forensic chemists, and toxicologists when handling these harmful substances!


|
IonSense, Inc. provides OpenSpot Mass Spectrometry
® solutions to the fields of food safety, forensics, drug development, and chemical analysis. We manufacture and develop direct analysis in real time (DART
®) technology licensed from JEOL USA, Inc. and atmospheric solids analysis probe (ASAP
®) licensed from M&M Consulting.
|
|
Copyright © 2014. All Rights Reserved.
|
|
|
|