WHAT'S NEW
BÜHLMANN Laboratories AG is proud to announce it has received FDA 510(k) clearance for its BÜHLMANN fCAL® turbo
The BÜHLMANN fCAL® turbo is an in vitro diagnostic assay intended for the quantitative measurement of fecal calprotectin, a neutrophilic protein that is a marker of intestinal mucosal inflammation, in human stool. The BÜHLMANN fCAL® turbo aids in the diagnosis of inflammatory bowel disease (IBD), specifically Crohn's disease (CD) and ulcerative colitis (UC) and aids in the differentiation of IBD from irritable bowel syndrome (IBS) in conjunction with other laboratory and clinical findings.
|
|
Educational Webinar
Given the high prevalence of gastrointestinal disease, the value of calprotectin in IBD patient diagnosis, and the downfalls of traditional treatment pathways, it is clear calprotectin testing is beneficial to patient care.
In this webinar, Dr. Arne Roseth shares in-depth knowledge on calprotectin and its utility in a clincal laboratory setting as well as his experiences with utilizing this biomarker to improve his patients care.
|
|
New BÜHLMANN fCAL® turbo Customer Testimonials
View a compilation of twelve user testimonials from UK, Ireland, Switzerland and France that have been published so far for the BÜHLMANN fCAL® turbo. Find valuable highlights, perspectives, advice and details about the successful applications of this automated calprotectin assay from each laboratory.
|
|
View this demonstration of quantitative trough level measurement for TDM using the Quantum Blue® TDM rapid test technology. (US:The Quantum Blue® tests are for Research Use Only. Not for use in diagnostic procedures. Quantum Blue® Adalimumab: Health Canada License: 101776;Quantum Blue® Infliximab: Health Canada License: 98838)
UPCOMING EVENTS
Stop by to visit BUHLMANN at AACC 2019. Connect with our team at Booth #4029 and learn about our BÜHLMANN fCAL® assay portfolio, Adalimumab and Infliximab assays, and more!
Visit the BUHLMANN Booth at AMLI 2019. Meet up with our team and learn about our autoimmunity, neuroimmunology, allergy, and calprotectin assays. Schedule a meeting or just pop in!
RESOURCES AND RESEARCH
New American College of Gastroenterology Guidelines
Ulcerative colitis (UC) is a chronic disease affecting the large intestine, in which the lining of the colon becomes inflamed and develops tiny open sores, or ulcers. This condition is the result of the immune system's overactive response.1 This is a disease with increasing incidence worldwide- nearly one million individuals each in the United States and Europe are affected by this condition and many more globally
|
|
CITATIONS
Latest IBDoc® Publications from ECCO 2019:
TECHNICAL NEWS
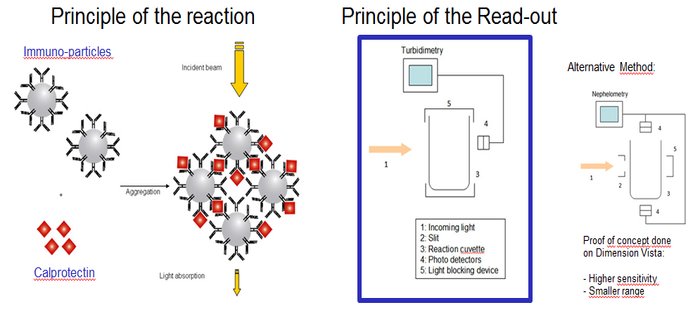
The Advantages of an Immunoturbidimetric Assay for Measuring Calprotectin in Fecal Samples
An immunoturbidimetric assay (or particle-enhanced turbidimetric immunoassay (PETIA)) for measuring Calprotectin is the fastest solution in the market with the shortest time to first result regardless of using sample batches or random access
BÜHLMANN HIGHLIGHTS
BÜHLMANN fCAL® turbo
BÜHLMANN fCAL® turbo, a turbidimetric immunoassay, is a flexible solution to be applied on most clinical chemistry analyzers. Turbidimetric technology is a milestone in fecal calprotectin quantification. This technology allows very rapid and flexible random access testing, and is the ideal solution for high throughput applications in clinical and research laboratories. Unique in speed, quality, and flexibility, this assay offers reliable information to aid clinicians in selecting patients for further diagnostic procedures.
BUHLMANN DIAGNOSTICS CORP
BÜHLMANN is unique, independent, and reliable in every aspect of its business from the outstanding quality of its products, its excellent after-sales services, and its remarkable scientific innovations. The establishment of BDC is a statement of the importance of you, our customers and our commitment to understanding your needs. Our North American affiliate will help us to continue to foster closer relationships with our customers and enhance our customer support interactions as well as meet your requirements for future BÜHLMANN products. Quality is not just a statement, but part of our daily mission.
105 Route 101A, Suite 1,
Amherst, NH 03031 USA
Ph: (844) 300-9799
info@buhlmannlabs.com
www.buhlmannlabs.com