 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
New Evidence of the Utility of Minimally Invasive Vaginal Biopsy
Dr. P. Yetur, L Worsch, S McClellan, D Flynn, and Dr. J. Tu from LabCorp with Hoag Hospital (Monrovia and Newport Beach, CA) and Bostwick Labs, (Uniondale NY) reported at the American Society of Colposcopy and Cervical Pathology / IFPC World Congress in Orlando (April 4-7th) presented new research on the utility of SoftBiopsy® and Spirabrush®. Specifically, they reported evidence that the use of these devices in the sampling of vaginal lesions seen under colposcopy after abnormal screening will result in less trauma and pain, while yielding diagnostic "trans-epithelial" samples in 94% of the 272 cases reported. A wide spectrum of pathological changes were diagnosed which ranged from normal to invasive squamous cell cancer. Thus, they conclude that vaginal biopsy can be painful using conventional punch forceps, while these new "bristle-brush or fabric-brush" devices offer a useful alternative during colposcopy.
|
 |
|
FAQs Answered By The Colposcopy Pros
|
Q : How does the Kylon® fabric biopsy work?
The shallow Kylon hooks do not penetrate the tissue when placed on it with pressure. Instead, when pressed and rotated onto the tissue, the hooks bend back at their junction with the base of the fabric until the hook tips contact the tissue surface, exposing the hook tip to the tissue, "activating" the hooks to remove tissue by applying frictional force to the surface. The de-bonding force creates buckling of the tissue just below the epithelium and excavates fragments identical to currentings (full thickness tissue strips) and fragments similar to small trans-epithelial biopsies.
Q: How do fabric based biopsy devices compare with sharp cutting tools or bristle brushes for biopsies of the exocervix and endocervix?
Exocervical Biopsy with the SoftBiopsy® Gynecological Biopsy Device that employs Kylon®: When pressure is maintained, the hooks rake across the tissue plane frictionally dislodging and shearing tissue strips and fragments from just under the basement membrane, which collect in the base of the fabric. Thus, the unique feature is the shearing away of tissue using frictional de-bonding forces. Multiple fragments or strips can be harvested many of which resemble multiple punch biopsy specimens, depending on the size of the platform the fabric is mounted on (SoftBiopsy® has a circular tip that is just under ½ inch in diameter). Therefore, the surface area covered is many times larger than the average punch biopsy tip. The device is disposable, the fabric functions as a tissue-trap container, and its tip is snapped off at the scored handle to be placed in a fixative vial for transport to the lab. The SoftBiopsy® is commercially sold as a cervical biopsy device and container system, and is registered with the FDA as a Class 1, 510k Exempt gynecologic biopsy device as well as a system for tissue collection, storage and transport.
|
 |
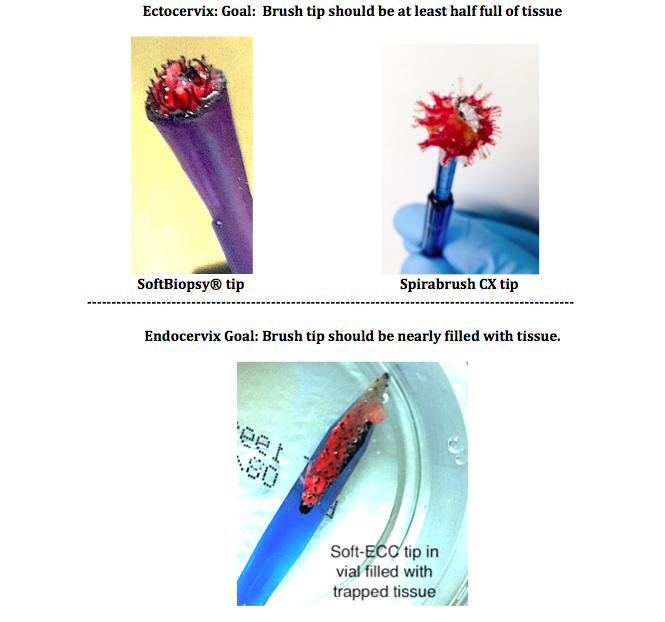
Inspect the bristles or hook arrays for trapped tissue prior to placement in the fixative vial. For endocervical fabric-based devices, the hook array should fill completely when used according to package insert guidance and proper fit or pressure on the endocervical mucosa during rotation. For extocervical frictional biopsy, with proper pressure and sufficient rotation, the device should fill within two applications (sites) on the cervix.
Inspect the device tip for sufficient trapped tissue prior to placement in the vial.
|
|
 |
Studies Prove the Effectiveness of Our Devices
Our minimally invasive devices are used by healthcare professionals nationwide. We know some of our customers are using our products within hospitals, multi facility health networks and practices with several providers. We encourage you to speak to your associates about the benefits of minimally invasive options to colposcopy. Our website is a great resource to share the research and product information that supports your decision to provide a gentle experience to your patients.
If your associates are interested in trying our devices, samples are available.
We also believe your comments would be invaluable to other providers who are considering using this technology. We would like to recognize the physicians and laboratories offering this innovation to women's health by posting your testimonial with your branding on our website. Your web posted comments would provide insight to other potential users and advertise your decision to offer a gentle patient experience.
Contact our Customer Service Department if you are interested in samples for your associates or would like to provide comments for web publication.
|
|
Gynecologic Biopsy Innovations
"Tissue based technologies to help
save lives worldwide"
|
We encourage you to share our product information with your associates. Feel free to forward!
|
 |
|
Soft-ECC
®
Endocervical Curette
|
|
SoftBiopsy
®
Gynecological Biopsy Device
|
 |
|

|
The FDA Compliant disposable Soft-ECC
® endocervical curette tissue collection system can be used to biopsy the endocervical canal during colposcopy or the evaluation of abnormal uterine bleeding. Unlike the conventional sharp endocervical curette, the Soft-ECC
® is intended to gently frictionally abrade and collect (trap) abundant trans-epithelial tissue samples into the patent pending KYLON
® fabric. The Soft-ECC-S
® employs a smaller pad and is designed for the short, shallow or stenotic cervix.
The fabric tip is detached, placed into the preservative vial, and transported to the lab where it is easily emptied for histological processing.
|
|
The FDA Compliant disposable SoftBiopsy
® tissue collection system can be used to biopsy the exocervix and lower genital tract during colposcopy or when a suspicious lesion is detected. Unlike the "sharp edge" biopsy design of gynecological biopsy devices, the SoftBiopsy
® design is intended to gently frictionally abrade and collect
(trap) abundant trans-epithelial tissue samples into the patent pending KYLON® fabric. The fabric tip is detached, placed into the preservative vial, and transported to the lab where it is easily emptied for histological processing.
|
|
|
|
SpiraBrush CX®
As Histologics continues to develop new innovations in women's health, we recognize the need for options. SpiraBrush CX
®, invented by the founders of Histologics, LLC, is one of the original devices developed for a minimally invasive approach to cervical biopsy.
During clinical settings such as colposcopy, a biopsy of the exocervix or lower genital tract may be indicated. If there is a suspicion of neoplasia, the SpiraBrush CX
® device, with a patented spiral shaped stiff bristle brush, can be used to collect tissue from the cervix as an alternative to other punch biopsy devices.
|
We believe you will understand the benefits of using
Soft-ECC
®, Soft-ECC-S
®, SoftBiopsy
®
& SpiraBrush CX®
devices
(includes three Soft-ECC®, one Soft-ECC-S® & three SoftBiopsy® & two SpiraBrush® devices, complete product information and use instructions,
Lab SOP instructions and Research Data)
Interested in bringing Soft-ECC®, SoftBiopsy®
or SpiraBrush CX® into your practice?
Please email: Histologics or call: 888-738-9757 or 714-81HISTO (714-814-4786)
|
Please use the links below for additional product and health related resources:
New! Video Summary of the "Compassionate" Value of Minimally Invasive Brush and Fabric-Based Tissue Sampling:
Product Information
|
Please contact us if you need additional information
or would like to place
an order:
Histologics LLC
4095 E. La Palma Ave., Ste. N
Anaheim, CA 92807
Toll Free Customer Support: 888-738-9757
Local: 714-81-HISTO
|
|
Copyright © 2017. All Rights Reserved.
|
|
 |
|
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
 |
|