|
Join Our List
 |
|
Robin Novak, RN, CIC
|
 |
|
Director of Infection Prevention
and
Endoscopy
636.875.5088 ext. 101
|
|
This Month's Training Opportunities
|
 |
November 9, 2016
Safe Injection Practices
Several new titles added this month.
|
|
Surveyor's Corner
|
 |
|
Cathy Montgomery, RN, CASC
AAAASF Surveyor
636.875.5088 ext 102
While admittedly not all surveyors are cut from the same cloth, I think we can all see the value in sharing citations as a way to continue baseline knowledge. Recently it came to my attention that an ASC was cited under Sanitary Environment for not dating the ABHS when opened. Evidently the surveyor was basing her citation on a published study that indicates "most hand sanitizers have a shelf life of approximately 3 years, which is indicated by the manufactures expiration date". However she added that hand sanitizers start evaporating once opened and are less effective in 6-12 months, thus the citation. The solution offered was to obtain written instructions from the manufacturer of your product to deny this stand, or date your ABHS when opened and discard at a 6 - 12 mo. window based upon your policy.
|
|
Recalls & Recent Events
|
|
Product Recall:
Nurse Assist is voluntary recalling all unexpired lots of I.V. Flush Syringes due to a potential link to Burkholderia cepacia bloodstream infections with the product. The lots being recalled were distributed to customers and distributors between February 16 and September 30, 2016.
Product can be identified
by the labeling on the packaging and device. Read the U.S. Food and Drug Administration
safety alert
.
CDC Issues Health Advisory on Contaminated Heater/Cooler Devices
|
|
|
|
Volume 1, Number 1
|
November 2016
|
 |
I am excited to present a rebirth of our Infection Prevention Newsletter and at the same time introduce our new Director of Infection Prevention, Robin Novak, RN, CIC!
Our newsletter has been appropriately renamed
ImPaCT and the goal is to offer monthly discussions by Robin on the infection prevention and control trends for ambulatory surgery centers.
ABOUT ROBIN NOVAK, RN, CIC
Registered Nurse, Certified Infection Preventionist dedicated to disease prevention and high quality healthcare. Strong decision maker who understands the importance of patient, visitor and staff safety following evidence based best practices.
Driven and compassionate healthcare professional with 30 years hands-on experience in fast-paced ambulatory surgery center and hospital environments. Accountable and responsible with an additional focus on Quality Assurance Process Improvement
.
In the past, Robin has served as the SGNA Carolina Chapter President, involved in SGNA Practice Committee as well as a Regional Committee member. Robin has been active with APIC and is a current member of APIC PALMETTO. Robin was prior employed at the Ambulatory Surgery Center of Spartanburg since 2004 and held roles of Endoscopy Nurse, Endoscopy Charge Nurse and most recently the Quality Assurance Process Improvement Coordinator. Robin has worked on several infection prevention projects for Excellentia Advisory Group including a key role as a faculty speaker at our annual Infection Prevention Strategies for ASC's conference in Las Vegas.
|
|
Infection Prevention in the ASC
|
By Robin O. Novak, RN, CIC
Today's regulatory climate requires ASC's to have a designated and qualified infection preventionist to direct its infection control program. This health care professional must be able to validate infection control qualification through training, but certification is not required. The lack of a designated individual will lead to a deficiency citation.
In addition to having a qualified individual, the facility must be able to demonstrate it has considered and adopted nationally recognized infection control guidelines. These guidelines will become the foundation of the infection prevention and control program. There are numerous professional organizations which have developed guidelines unique to a specialty. Some of the organizations include AORN, SGNA, AAOS, and AAMI, etc. There are also the more generic and broad guidelines of the CDC/ HICPAC.
Guidelines are scientific- researched, evidence-based, fundamental principles which provide foundational protocols for a facility infection control and prevention program. Some of the most common guidelines include:
1) Guideline for Isolation Precautions (CDC/HICPAC)
2) Hand hygiene (CDC/ HICPAC)
3) Disinfection and Sterilization in Healthcare Facilities (CDC/HICPAC) and
4) Environmental Infection Control in Healthcare Facilities (CDC/HICPAC).
For surgery centers, AORN has the Perioperative Standards and Recommended Practices which are updated yearly to reflect current evidence-based practice. SGNA is the leading influencer for anything related to endoscopy. AAOS offers clinical practice guidelines for the orthopedic specialty surgery centers.
|
| Critter Craze |
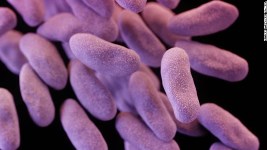
By Robin O. Novak, RN, CIC
If you have been involved in the healthcare industry anytime in the past year, you have heard of the duodenoscope outbreak caused by CRE organism. Literature suggests the CRE outbreak was not a result of a lapse in infection prevention and reprocessing standards but may have been related to device design.
CRE are resistant to most commonly used antibiotics. This resistance makes them very difficult to eradicate and thus a threat to public health.
Escherichia coli (E. coli) and Klebsiella species are bacteria found in the normal human intestines. Sometimes, these bacteria can multiply outside of the digestive tract and cause significant infections. Because of that, the Enterobacteriaceae are the most common cases of bacterial infections in both healthcare and community settings.
Typically, healthy people do not get CRE infections as they have immune systems which can fight the bacterial overgrowth. However, patients being treated for another condition are at greatest risk for contracting a CRE bacterium. Also, patients with immune-compromised systems and those undergoing invasive procedures such as ERCP have an increased risk.
Transmission of CRE is typically spread person-to-person through contact with infected people. But, as mentioned earlier, some medical devices such as intravenous catheters, urinary catheters and endoscopes can cause the CRE infection. Finally, CRE can also cause infection in wounds resulting from traumatic injury or surgery.
The treatment for CRE infection should be individualized. Early on, healthcare teams should determine if the patient is colonized versus infected. If the patient is colonized, treatment may not be necessary. However, for those that have an infection, it is important to note that CRE is resistant to commonly prescribed antibiotics (Centers for Disease Control and Prevention [CDC], n.d.).
References
Centers for Disease Control and Prevention. (n.d.). Healthcare-associated Infections (HAIs). Retrieved from
http://www.cdc.gov/hai/organisms/cre/cre-patientgeneral.html
|
| GI Corner |

By Robin O. Novak, RN, CIC
Recent changes to AAMI ST-91, AORN, and SGNA endoscope reprocessing documents have awoken the sleeping bear otherwise known as the GI Community. Like never before, there has been open dialogue about reusable accessories commonly reprocessed for the lifetime of the accessory.
What is the hubbub all about? The newest guidelines recommend the reusable accessories and valves should be reprocessed and stored as a set with the endoscope to improve traceability.
ASC's and GI facilities are scrambling to meet the new standards. The greatest concerns seem to revolve around a couple of issues. 1) How can individual valves and accessories be reprocessed as a unique set in an AER? 2) Does the new standard provide recommendation about storage options? 3) Once a scope is in use with the unique set and one of the valves fail, what do you do about replacing that valve? Do you then have two valves assigned to the single endoscope?
Sadly, there are no easy answers. Each individual facility will need to evaluate their options based on the types of equipment used at their facility. Some facilities have chosen to move forward using mesh bags or specialized containers to secure the accessories. Others are investigating the option of disposable accessories.
There are caveats with both methods. The use of either method may require additional storage. While the use of disposable supplies may increase cost or they may not be available for the type of endoscope at your facility.
If you would like a cost analysis of disposable versus re-usable valves for your facility, reach out to Excellentia Advisory Group.
|
|
|
If you are in need of assistance with your infection prevention program, Robin will be able to assist with everything from setting up your program, training your Infection Preventionist, writing or editing Policies & Procedures or just simple telephonic consultation. Please let me know how we can help.
Sincerely,
Cathy Montgomery
Excellentia Advisory Group
[email protected]
636.875.5088 extension 102
See what's happening on our social sites.
|
|
|