Sav-Mor Pharmacy Services Weekly Newsletter | |
Core Member Weekly Newsletter & Updates | |
September 18th - 22nd Weekly Update | |
|
eTrueNorth COVID Bridge Access Program
eTrueNorth is excited to partner with retail pharmacies to offer COVID-19 vaccine services in your community. Pharmacies enrolled in the Bridge Access Program (BAP) with eTrueNorth will receive reimbursement for administering COVID-19 vaccines to uninsured and underinsured adults.
Sign up with eTrueNorth to bring COVID-19 vaccinations to your community. Through the Bridge Access Program, pharmacies enrolled with eTrueNorth can administer no-cost COVID-19 vaccines to uninsured and underinsured adults 18 and older.
The Sav-Mor Corporate office is currently in the process of credentialling our stores to participate in this program. Should you wish to be proactive and sign your pharmacy on its' own - see the link below!
| |
|
Pfizer COVID Vaccine Available for Early Shipping
Update: All current Pfizer COVID Vaccine orders have been distributed and Cardinal Health has additional capacity to ship Pfizer Adult Single Dose Vials (CIN 5860424) now. If ordered this week, products can be expected to ship next week for delivery.
Ordering COVID-19 Vaccines
Orders for COVID-19 vaccines can be placed through Cardinal Health’s Flu/COVID Care portal,
accessible in Order Express. A Specialty Pharmaceutical Distribution (SPD) pass through account
is required to place and confirm vaccine orders for purchase. Orders will ship via FedEx, first
come, first serve, from our specialty warehouse in LaVergne, TN. Orders should NOT be placed
for the full amount of product needed for the season, as COVID vaccine products can be
ordered weekly through the Flu/COVID Care portal.
If you have any questions, please reach out to the Flu Team via email, FluTeam@cardinalhealth.com, or call 1.833.358.8326 option 3, option 2.
| |
|
DSCSA Implementation: LSPedia
On August 25th, the FDA issued a one-year delay on the implementation of DSCSA (Track & Trace) that was set to take effect November 27th, 2023. It is important to announce that while the FDA will not enforce the implementation, state board inspectors can and will still enforce some requirements as mandated by November 27th, 2023.
All retail pharmacies are still required to comply with the existing DSCSA requirements, including:
- Validating your Authorized Trading Partners;
- Accepting products that possess the Product Identifier (NDC, Serial Number, Lot Number and Expiration Date) in human and machine-readable formats (2D Barcode);
- Receiving and verifying the transaction data (transaction information, transaction Statement, and transaction History (collectively known as the 3Ts) for all DSCSA Covered Products; and
- Continuing due diligence to identify suspect products and incorrect transaction data, conduct investigations, respond to information requests, and notify the FDA when you identify Illegitimate products
LSPedia & Sav-Mor Partnership:
To support Sav-Mor members and independent pharmacies, LSPedia is offering FREE DSCSA compliance until 11/1/2023 and the ability for Pharmacy Pro customers to pause payments from 11/1/2023 to 6/1/2024 for all independent pharmacies who sign up before 11/27/2023. This allows all pharmacies to have a system in place, compliant with both current and future DSCSA requirements. Our system allows pharmacies to receive both T3 data via ASN EDI (current requirement) and EPCIS (11/24/2024 requirement) along with the many other Pharmacy Pro features.
Sav-Mor has negotiated discounted pricing of $84/month for the Pharmacy Pro Package. If you sign up prior to 11/27/2023 – you can pause payments for a year. This is a great value for our members!
Sign Up Today! https://www.lspedia.com/onescan-pharmacy-registration
Discount Code: LSPSAVMOR
| |
Sav-Mor Partnership AI-Cash Pricing | |
|
Want to try to bypass the discount card craze? Use Prescryptive Health
Sav-Mor Pharmacy Services has partnered with Prescryptive Health to bring artificial-intelligence-optimized pricing to its members at discounted rates in a new PSAO-level contract. Prescryptive’s state-of-the-art AI Pricing solution can make community pharmacies immediately more competitive by giving them access to cash pricing optimization driven by a predictive model that consistently adjusts based on evolving analytics.
For more information on the benefits to your business and customers, or to schedule a demo to see how Prescryptive AI Pricing works, please reach out to Scott Ousley. To get started, simply complete the short form!.
Scott Ousley | Director Business Development
Email: Scott.Ousley@Prescryptive.com
Cell: (918) 984-5370
www.prescryptive.com
| |
|
Recent PBM Communications Requiring Mail Order Sent to Patients
This week a member shared a communication that a patient had received from their PBM. This letter stated that their medications were now required to be filled at the PBM's affiliated pharmacy, or by mail according to the plan. The letter also gave instructions on how to transfer. Sav-Mor wanted you all to be aware in case similar communications are being sent to your patients. Our recommendation would be to request copies of those communications and send them directly to your local State Representative in Lansing, as well as your representatives in Washington.
| |
|
Urge Your Representative to Vote Yes on PBM Reform and Transparency Legislation
Please take a moment and contact your Representative and urge them to support H.R. 5378, the Lower Costs, More Transparency Act, which we anticipate could come to the floor for a vote in the House of Representatives as early as next week.
H.R. 5378 is the result of a month’s long process to combine the efforts of the House Energy and Commerce, Ways and Means, and Education and the Workforce committees into a single legislative package.
| |
|
Michigan Senate Bill (SB) 219 has been officially signed by Gov. Gretchen Whitmer effective July 19th, 2023.
SB 219 authorizes pharmacists to independently prescribe and administer Advisory Committee on Immunization Practices-indicated (ACIP) vaccines to patients ages 3 and up. Additionally, it allows for pharmacists to independently administer Clinical Laboratory Improvement Amendments-waived (CLIA) tests for influenza and COVID-19. In the event of a positive test, the legislation allows pharmacists to dispense appropriate antiviral therapies to the patient.
The final version of the bill includes the following provisions:
- Pharmacists may independently order an immunization recommended by ACIP to individuals 3 years of age and up provided they have completed a board-approved training program.
- Requires pharmacists to provide information about the Vaccines for Children Program to individuals younger than 19 years of age.
- Requires pharmacists to report all immunizations they administer into MCIR.
- Pharmacists may independently order and administer a CLIA-waived test for COVID-19, influenza, or other respiratory illness provided they have completed a board-approved training program. This statutory authority does not preempt a pharmacist’s ability to order and administer CLIA-waived tests as otherwise authorized under federal law or pursuant to a collaborative practice agreement.
- Based on the result of a COVID-19 or influenza test, pharmacists may dispense antiviral therapy to a patient without a prescription.
| |
Want to know how to get a CLIA WAIVER?
-
Complete the application. This should take no more than 10 minutes. We highly recommend that you view our guidance document as well as NCPA's useful video for help on each part of the application.
-
Submit your completed application to the specific agency in your state that processes them. You can use this CMS document to find the email address or FAX number for the agency for submitting your application.
-
Do not send payment with the application. After your application is processed and approved by your state agency, you will receive an invoice (CLIA user fee coupon) for $150 from CMS in Maryland. Once the invoice is paid, CMS will mail your CLIA certificate. The entire process usually takes 4-6 weeks, but of course can vary from state to state depending on volume of applications and resources.
- Your CLIA certificate will be valid for a period of two years from the date of issuance.
| |
2024 Medicare Part D - Ensure that your pharmacy is aware of 2024 changes! | |
|
Prepare for 2024 Changes to DIR Collection & Cash Flow!
Higher up-front DIR fees coupled with lower patient copays can cause financial pain in Q1 and Q2. See our resources below to see how to ease the pain and stay ahead of the game.
What can I do to prepare?
It is advised that your pharmacy start to track how much DIR is being deducted from each (PBM) in 2023 and begin to accrue funds for this transition period to avoid potential disruptions in cash flow.
| |
Audit Tips - Stay Up-To-Date on Pharmacy Audits! | |
|
Audit Tip of the Month: Recent DEA Rule Change - Partial Fills for Schedule II Controlled Substances
The DEA recently updated the rule regarding partial fills of Schedule II (C-II) prescriptions. The change addresses regulations such as how the prescribing practitioner should indicate the C-II should be partially filled, and how the pharmacist should record the partial fillings.
Prescriber Requested
The DEA rule clarifies that a prescriber “must specify the quantity to be dispensed in each partial filling on the face of the written prescription, in the written record of the emergency oral prescription, or in the record for an electronic prescription”. Additionally, a pharmacist may contact the prescriber after receiving a prescription without a partial fill annotation if the pharmacist believes a partial fill is appropriate, but they do not wish to seek approval from the patient. If the prescriber authorizes the partial fill, “the pharmacist must note the following: “Authorized by Practitioner to Partial Fill,” the name of the practitioner, the date and time of the discussion, and the pharmacist's initials”.
Patient Requested
The rule also clarifies that the patient, a parent or legal guardian of a minor, or an adult patient’s medical power of attorney may request a partial fill. Their request to partial fill may be received via phone or by sending a signed written note to the pharmacy with a family member. For partial fills, the pharmacist must record “(1) “The [patient, parent or legal guardian of a minor patient, or caregiver of an adult patient named in a medical power of attorney, whichever is applicable] requested partial fill on [date such request was made],” and (2) the quantity dispensed”.
When partial filling C-II prescriptions, the DEA clarified that the pharmacist must record the following elements:
-
Quantity Dispensed: On the face of the written prescription, in the written record of the emergency oral prescription, or in the record of the electronic prescription -OR- the recordkeeping for a written prescription or an emergency oral prescription can be maintained in the pharmacy’s electronic recordkeeping system
- Caution: if the partial fill is not pursuant to the prescriber or patient’s request, but instead pursuant to the pharmacy’s inability to supply the full amount, an LTCF patient, or a terminally ill patient, then documentation must occur on the face of the written prescription, in the written record of the emergency oral prescription, or in the record of the electronic prescription
-
ALL Partial Fills: The pharmacy must have a record with “the date of each dispensing, the name or initials of the individual who dispensed the substance, and all other information required by 21 CFR 1306.22(c) for schedule III and IV prescriptions” such as:
- drug name
- dosage form
- date filled/refilled
- quantity dispensed
- initials of dispensing pharmacist for each refill
- total number of refills for that prescription
- Note: For an electronic prescription, the quantity dispensed, date dispensed, and the dispenser must be linked to the electronic prescription
| |
|
Due to more stringent PBM credentialing requirements, all payors are now require NCPDP Part II to be completed by the pharmacy. This includes uploading the current General and Professional Liability Certificates, as well as the PIC license. Once Part II is complete, the profile will be assigned a Credential Date. This is the date the pharmacy indicates (by checking a box on the “Verify and Submit” page) that they have reviewed their profile and made sure all required fields and uploaded documents are correct and current for credentialing purposes; therefore, confirming the profile and documents are up to date. A Part II training guide is available on NCPDP.
Since all PBMs utilize NCPDP for credentialing purposes, the profile should always be current, with a Credential Date indicating the profile has been updated within the last 12 months. When updating the information, it would be an excellent time to complete and sign the 2023 FWA Attestation.
Please reach out to Charisse.Carpenter@sav-mor.com with any questions
| |
|
Express Scripts 2024 Medicare Part D Networks - Update
What is happening?
In preparation for the 2024 Medicare plan year, Cardinal Health PSAOs, at the direction of the PSAO Advisory Panel, have declined participation in two Express Scripts Medicare Networks, Medicare Part D Premier Preferred Performance Network (MEDPPN) and Medicare Part D Regional Preferred Performance Network (MEDRPPN). The Express Scripts MEDPPPN and MEDRPPN networks will be utilized by plan sponsors as their preferred provider network. The decision to decline participation in this network was based on the inflexible position of Express Scripts when negotiating reimbursement rates that the Advisory Panel deemed below acceptable reimbursement.
What does this mean for my pharmacy?
In the current 2023 plan year, Cardinal Health PSAO members have preferred access to several plan sponsors through Express Scripts. In 2024, PSAO members will have access to all Express Scripts plan sponsors as non-preferred providers.
The Advisory Panel has accepted the terms of participation for the Medicare Part D National Performance Network (NPN). This network, along with the ES1000-MedD network, will be utilized for broad access for Medicare Part D Networks.
Express Scripts, at their discretion, may solicit Cardinal PSAO members directly to participate in MEDPPN or the MEDRPPN networks.
What action do I need to take?
If your pharmacy receives a solicitation to participate in one or both Medicare Part D Networks, your pharmacy should scrutinize the agreement when evaluating whether to participate. Be sure to consider the financial impact of participation as preferred provider compared to a non-preferred provider in determining the best course of action for your pharmacy.
If a member pharmacy does not receive a solicitation for the ESI-MEDPPN or the ESI-MEDRPPN networks, no action is needed.
As a reminder, all PSAO agreements can be reviewed through the contract portfolio. Additional 2024 Medicare Part D information will be communicated in the coming weeks as the Open Enrollment period approaches.
| |
|
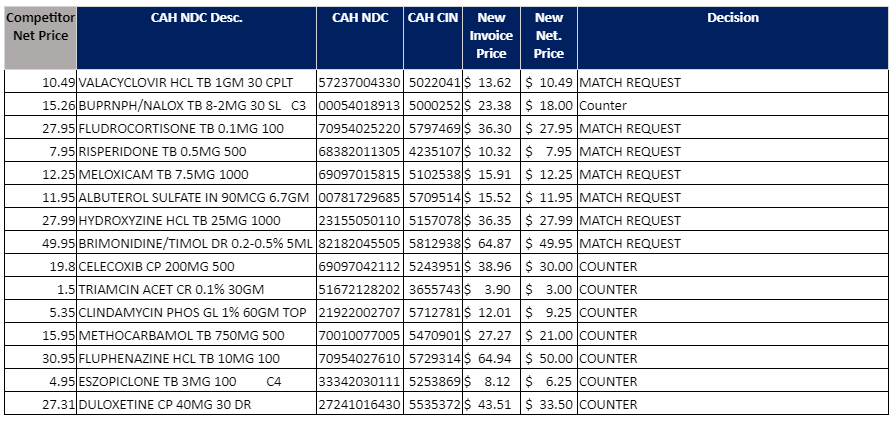
New Cardinal Pricing Agreement Started August 1st
We are a full month into the new pricing agreement with Cardinal and the response has been outstanding! In August our members were able to bring almost $100,000 in generic drugs back to the group because of the larger generic rebates. This, combined with carving out GLP1 drugs led to nice increases in compliance, which will lead to even larger savings starting October 1st when the new Cost of Goods kicks in and we see lower costs on brand name (non-specialty/GLP1) drugs!
Below are items that the Cardinal Generics team recently adjusted the prices on based on the competitive information supplied by membership. These prices are now active in Order Express. Please note that all overrides are now set with an Invoice price, and are now rebated down to the net price listed. I have another set of items that I am currently working on the with the Generics team that should be available next week. I will send an update when that is complete. As always, thank you all for your help.
|  | | | | |